 PDF(1961 KB)
PDF(1961 KB)


 PDF(1961 KB)
PDF(1961 KB)
 PDF(1961 KB)
PDF(1961 KB)
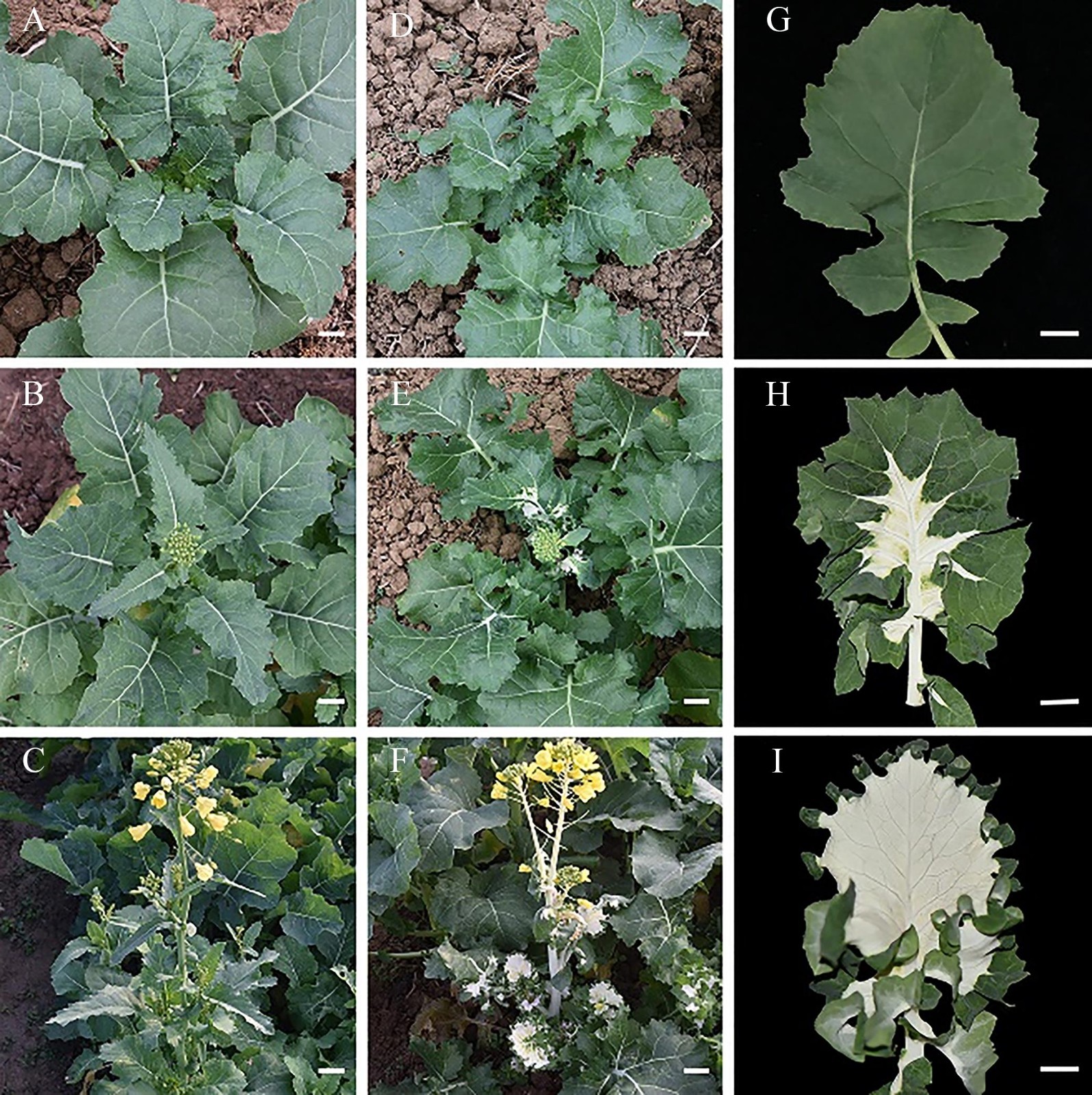
甘蓝型油菜叶片白化分子机理研究
Molecular Mechanisms of Albino Leaves in Brassica napus
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |