 PDF(916 KB)
PDF(916 KB)


 PDF(916 KB)
PDF(916 KB)
 PDF(916 KB)
PDF(916 KB)
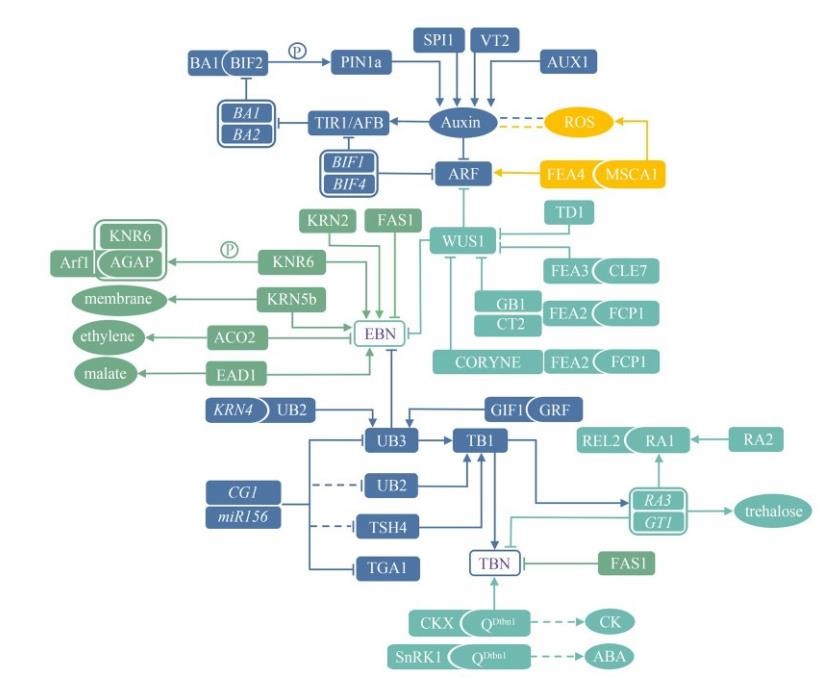
玉米花序分枝和穗粒数发育的分子调控
Molecular Regulation on the Development of Reproductive Branches and Kernel Number in Maize Inflorescences
| {{custom_ref.label}} |
{{custom_citation.content}}
{{custom_citation.annotation}}
|
/
| 〈 |
|
〉 |